Enthalpy of Neutralization of Hcl and Naoh Value
The heat of neutralisation between hydrochloric acid and sodium hydroxide solution is -4998 kJ mol-1. Thermochemistry determine the heat exchanged at constant pressure q m c T.

Enthalpy Of Neutralization Of Hcl By Naoh Is 55 84 Kj Mol And By Nh4oh Is 51 34 Kj Mol The Enthalpy Of Ionization Of Nh4oh Is
The molar enthalpy change for the neutralization reaction.

. Q m x cp x T Q 50 x 418 x135 Q 28215 J Step 2. Volume of solution 250 250 mL 500 mL. One source that provides the enthalpy shift of sodium hydroxide solution neutralization with HCl as-579 kJ mol-1.
G NaOH418 Jg C400C - 200 C 8360 J. Q mcΔT 345 g x 41814 J. Calculating the limiting reactant the change in enthalpy of the reaction H rxn can be determined since the reaction was conducted under conditions of constant pressure.
Q34 The enthalpy of neutralisation of HCl and NaOH is -57 kJ mol-. The limiting reactant is either the HCl or the NaOH since there are equimolar amounts present. This demonstration is usually performed when topics in thermochemistry or thermodynamics are being discussed.
For example if you start with 345 grams of hydrochloric acid at 26C and its temperature increass to 291C when you add sodium hydroxide to it compute the heat of neutralization as follows. The heat capacity of the calorimeter is 279 JC. Q solution 50.
For example one source which gives the enthalpy change of neutralisation of sodium hydroxide solution with HCl as -579 kJ mol-1 gives a value of -561 kJ mol-1 for sodium hydroxide solution being neutralised by ethanoic acid. HNO 3aq NaOHaq NaNO 3 aq H 2 Ol ΔH -573 kJ. Calculate the number of moles of HClaq NaOHaq -- NaClaq H 2 Ol Energy.
You combined solid NaOH with dilute aqueous HCl. Use the density of water again to approximate the mass of HCl. To estimate the enthalpy of neutralization of hydrochloric acid and sodium hydroxide.
Furthermore what is the enthalpy of neutralization of HCl and NaOH. HCl aq NaOH aq NaCl aq H20 I is AH -573 kJmol. Heat of neutralization of NaOH and HCl is -5746kJequivalent.
For example if you start with 345 grams of hydrochloric acid at 26C and its temperature increass to 291C when you add sodium hydroxide to it compute the heat of neutralization as follows. The temperature rose from 298 K to 3174 K. Q mcΔT 345 g x 41814 J g x C x 31C 44748 Joules.
For example one source which gives the enthalpy change of neutralisation of sodium hydroxide solution with HCl as -579 kJ mol-1 gives a value of -561 kJ mol-1 for sodium hydroxide solution being neutralised by ethanoic acid. Click to see full answer. The calculated enthalpy shift of neutralization for reactions involving acetic acid or ammonia is a few kJ less exothermic than with solid acids and bases.
The temperature increased 135oC. What Is The Enthalpy Of Neutralization Of Hcl And Naoh. What is the enthalpy of neutralization of HCl and NaOH.
H OH H 2 O 137 kcal. For very weak acids like hydrogen cyanide solution the enthalpy change of neutralisation may be much less. By the law of conservation of energy.
The heat released by this reaction can be calculated with the equation q nAH where n is the number of moles of the limiting reagent. The energy released by the reaction is q reaction. By the law of conservation of energy.
Calculate the energy change for the amount of reactants in the calorimeter. Enthalpy of Neutralization for HCl NaOH 75 x 10joulegram x 100g - 173 x 10joulegram x 100g -75kJmol HCl 75kJmol NaOH. Of neutralization of HCl and NaOH.
Q reaction q solution 0 q reaction -q solution -8360 J. Q solution 50. NaOHaq HClaq Na aq Cl aq H 2 O.
The thermochemical equation for the reaction between nitric acid and sodium hydroxide solution is as shown below. H aq Cl aq Na aq OH aq. Thermochemistry determine the heat.
NaOH HCl NaCl H₂O. The limiting reactant is either the HCl or the NaOH since there are equimolar amounts present. The heat evolved at constant pressure in kJ when 05 mole of HSO react with 075 mole of NaOH is equal to 64 A 57 x B 57 x 05 057 D 57x025.
HClaq NaOHaq NaClaq H 2 Ol ΔH 57 KJ. H rxn q rxn moles of limiting reactant. For example if we use the values for water given earlier we can calculate the enthalpy of neutralization for hydrochloric acid and sodium hydroxide.
Add the mass of solid NaOH and the mass of HCl to give the total mass used. Calculate the enthalpy change per mole of HCl. HClaq NaOHaq -- NaClaq H 2 Ol Energy.
For example in the neutralization of HCl and NaOH. For example one source which gives the enthalpy change of neutralisation of sodium hydroxide solution with HCl as -579 kJ mol-1 gives a value of -561 kJ mol-1 for sodium hydroxide solution. The specific heat capacity is the same as water 418 JK g.
For example one source which gives the enthalpy change of neutralisation of sodium hydroxide solution with HCl as -579 kJ mol-1 gives a value of -561 kJ mol-1 for sodium hydroxide solution being neutralised by ethanoic acid. Moles of HCl 00250 L HCl 0700mol HCl1L HCl 00175 mol HCl. The specific heat for reaction 3 can be assumed to be close to that of pure water 4184 JgC.
The chemical reaction is given below. Y-axis-temperature change x-axis- volume of NaOH A graph of temperature against volume of sodium hydroxide solution was drawn. The energy released by the reaction is q reaction.
G NaOH418 Jg C400C - 200 C 8360 J. How do you calculate the enthalpy of neutralization of HCl and NaOH. G HCl 50.
This is an exothermic reaction which means heat is released. How do you calculate the enthalpy of neutralization of HCl and NaOH. Enthalpy of neutralization is the heat evolved when one gram equivalent of the acid is completely neutralized by a base in dilute solution.
G HCl 50. What is the molar enthalpy of neutralization per mole of HCl. The equation for the reaction is.
Find the enthalpy of neutralization of HCl and NaOH. The reaction of HClaq a strong acid with NaOHaq a strong base is an exothermic reaction The big idea for most calorimetry themed demonstrations is energy is conserved. In general the heat capacity of chemicals varies depending on how they are stored and.
87 cm3 of 16 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 16 mol dm-3 NaOH. For example one source which gives the enthalpy change of neutralisation of sodium hydroxide solution with HCl as -579 kJ mol - 1 gives a value of -561 kJ mol - 1 for sodium hydroxide solution being neutralised by ethanoic acid. Polythene bottle 250 ml with mouth Rubber cork with two holes to fit in the mouth of polythene bottle thermometer 110th degree stirrer with a.
Q reaction q solution 0 q reaction -q solution -8360 J. Enthalpy heat of neutralization 5 A graph of temperature change against volume of sodium hydroxide solution 0 2 4 6 8 10 12 14 16 18 20 0 05 1 15 2 25 3 35 4 45 5 55 6 Y-Values Key.

Enthalpy Of Neutralization Of Hcl By Naoh Is 55 84 Kj Mol And By Nh4oh Is 51 34 Kj Mol The Enthalpy Of Ionization Of Nh4oh Is

Enthalpy Of Neutralization Of Hcl By Naoh Is 55 84 Kj Mol And By Nh 4 Oh Is 51 34 Kj Mol Youtube
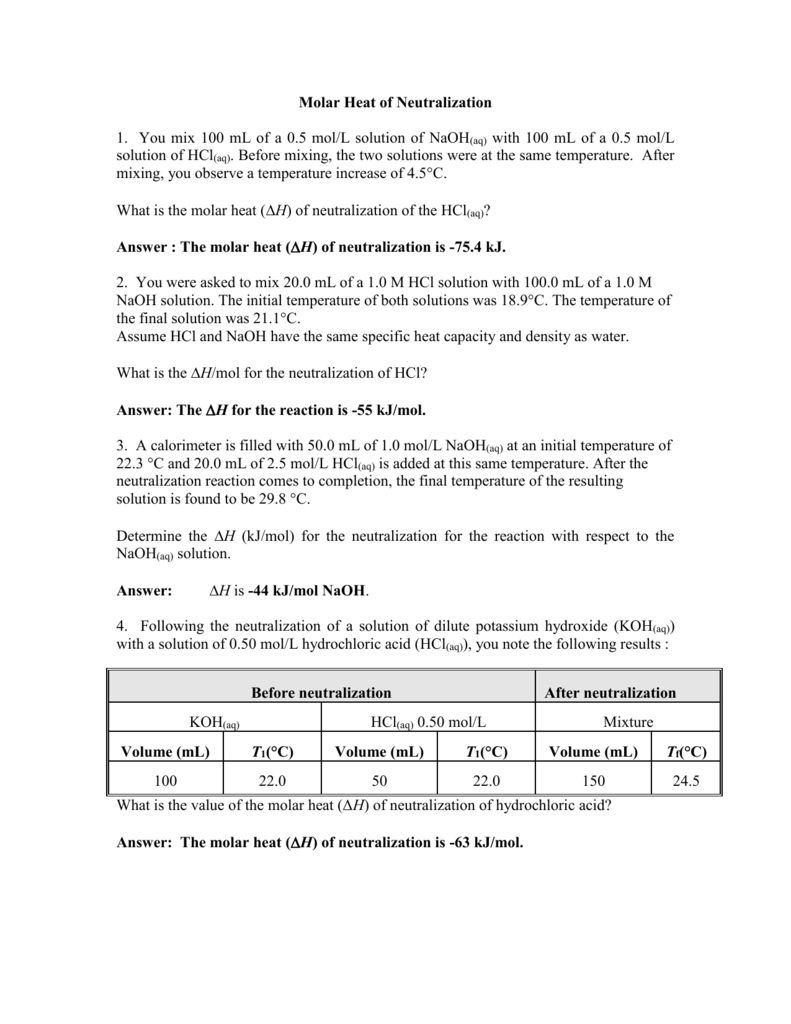
No comments for "Enthalpy of Neutralization of Hcl and Naoh Value"
Post a Comment